Why is ANKTIVA® Right for You?

ANKTIVA is an Immunotherapy that Activates Your Body’s Cancer Fighting Cells
ANKTIVA is the first U.S. FDA-approved immunotherapy that activates a type of cell called a natural killer (NK) cell, part of the body’s natural immune system, to attack and kill non-muscle invasive bladder cancer (NMIBC) cells.1 ANKTIVA is a treatment for use in combination with a standard treatment for NMIBC, bacillus Calmette-Guérin (BCG), for people with NMIBC for whom BCG alone was not effective or in whom NMIBC returned after initial successful treatment. These people have what is termed BCG-unresponsive NMIBC. ANKTIVA plus BCG is an option for people with BCG-unresponsive NMIBC carcinoma in situ (CIS) who are unable or unwilling to have their bladder surgically removed.
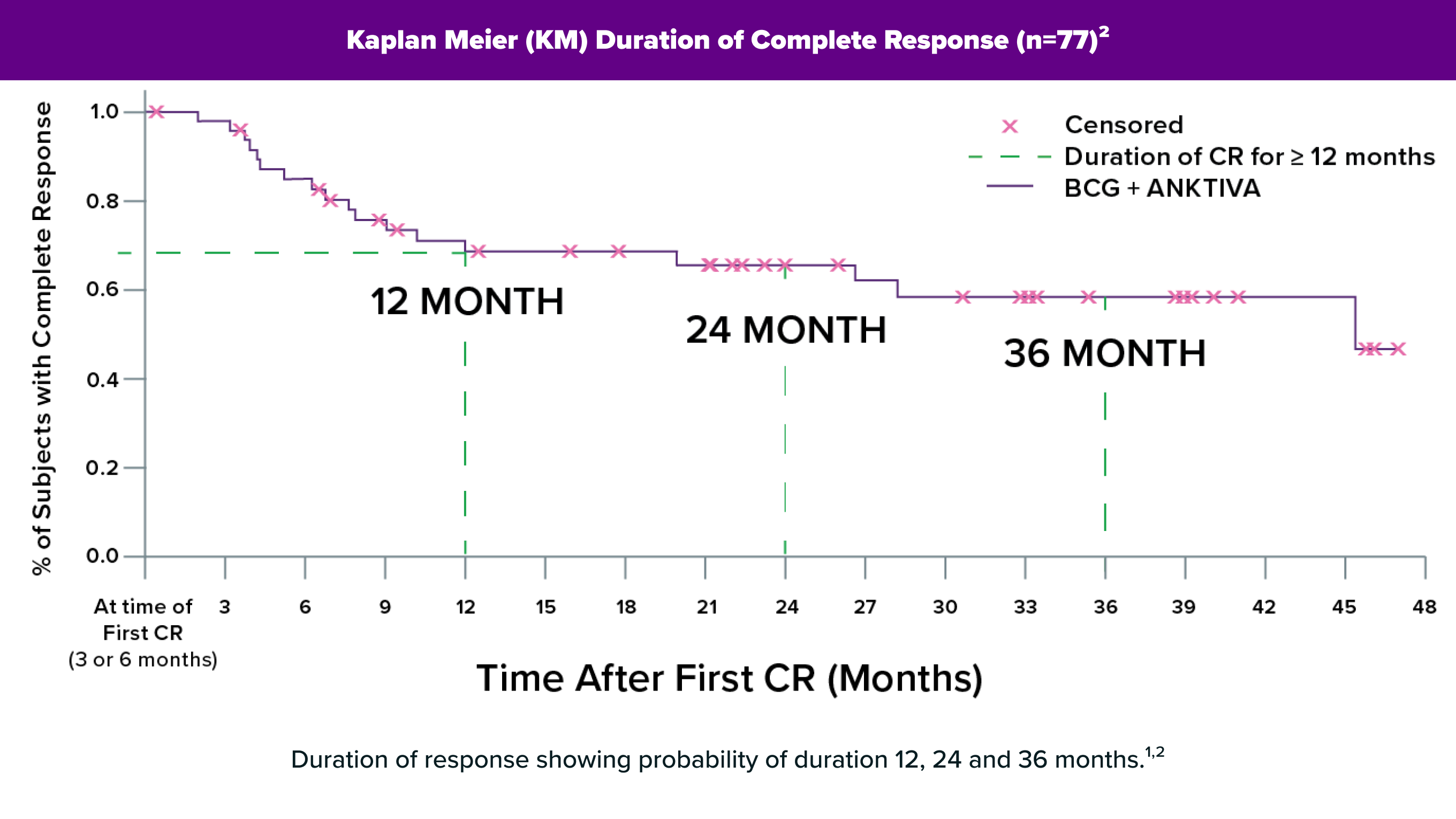
of study participants had a complete response, meaning their cancer was eliminated1
(n=100)(95% CI=61.1,79.6)
Duration of Response, meaning some study participants remained NMIBC-free for over 4 years1
1. Chang, S. (2025, April 26-29). An Update on QUILT-3.032: Durable Complete Responses to NAI (ANKTIVA) Plus BCG Therapy in BCG-Unresponsive CIS With or Without Ta/T1 Papillary Disease and in Papillary Disease without CIS. [Conference Presentation]. AUA2025, Las Vegas, Nevada, United States.
Who Should Consider ANKTIVA?
ANKTIVA is a treatment for adults who have all the following:
- High-risk non-muscle invasive bladder cancer
- NMIBC that did not or is no longer responding to BCG therapy alone (BCG-unresponsive)
- NMIBC that has not grown beyond the inner lining of the bladder to the muscle underneath (called carcinoma in situ or CIS disease)
- NMIBC with or without small finger-like tumor growths that project away from the bladder wall into the bladder (papillary disease)
If You Have Been Diagnosed with BCG-Unresponsive NMIBC CIS Disease and are Interested in ANKTIVA, Here is What You Need to Know:
What ANKTIVA is
- ANKTIVA is an immunotherapy that activates your body’s cancer fighting cells
- ANKTIVA is used in combination with BCG and is administered directly into your bladder via a catheter
- ANKTIVA is the first treatment of its type approved to treat BCG-unresponsive NMIBC
- ANKTIVA can be administered in a clinical setting and allows you to remain in the care of your urologist
What ANKTIVA is not
- ANKTIVA is not a chemotherapy or gene therapy
- ANKTIVA is not administered via an IV and does not circulate throughout your body. It only fights cancer in the bladder. This may reduce side effects
- ANKTIVA is not a chemical, it is a biologic that is similar to molecules produced naturally by your body
- ANKTIVA does not require you to change providers for treatment

Getting Started with ANKTIVA for Patients
A patient-friendly overview of how ANKTIVA plus BCG work together, as well as what patients should expect before and after treatment
Patient Stories
Read about patients who participated in the clinical trials for ANKTIVA
Justin’s Journey
An avid outdoorsman, Justin faced a challenging diagnosis of non-muscle invasive bladder cancer. After struggling with the side effects of his Bacillus Calmette-Guérin (BCG) treatment, he found renewed hope though his doctors' introduction to an immunotherapy for those who experienced a recurrence of NMIBC.
Wayne’s Journey
Wayne has lived a rich life with his wife, two daughters, and three grandchildren. He describes himself as someone who “just likes people.” When he was first told, “you might have a tumor in your bladder,” he became very concerned, especially when he learned his first treatment with BCG wasn’t working. Wayne feels lucky that a friend told him about a clinical trial for patients like himself who were not cured by BCG alone.
ANKTIVA is a Novel Treatment for NMIBC CIS Patients Unresponsive to BCG
For more information, please call 1-877-ANKTIVA