FAQs
What is ANKTIVA®?
ANKTIVA, also known as N-803, is a novel immunotherapy, different than an immune checkpoint inhibitor, that enhances the body’s natural immune response by activating natural killer (NK) cells and T-cells. It is designed to improve the effectiveness of immune-based treatments, including treatment of BCG-unresponsive non-muscle invasive bladder cancer carcinoma in situ.
How does ANKTIVA work?
ANKTIVA works by targeting the IL-15 pathway, which is critical for the activation and proliferation of NK cells and T cells. By amplifying the immune response, ANKTIVA enhances the body’s ability to recognize and destroy cancer cells while preserving healthy tissue.
Is ANKTIVA FDA-approved?
ANKTIVA is FDA-approved for BCG-unresponsive non-muscle invasive bladder cancer carcinoma in situ with or without papillary tumors.
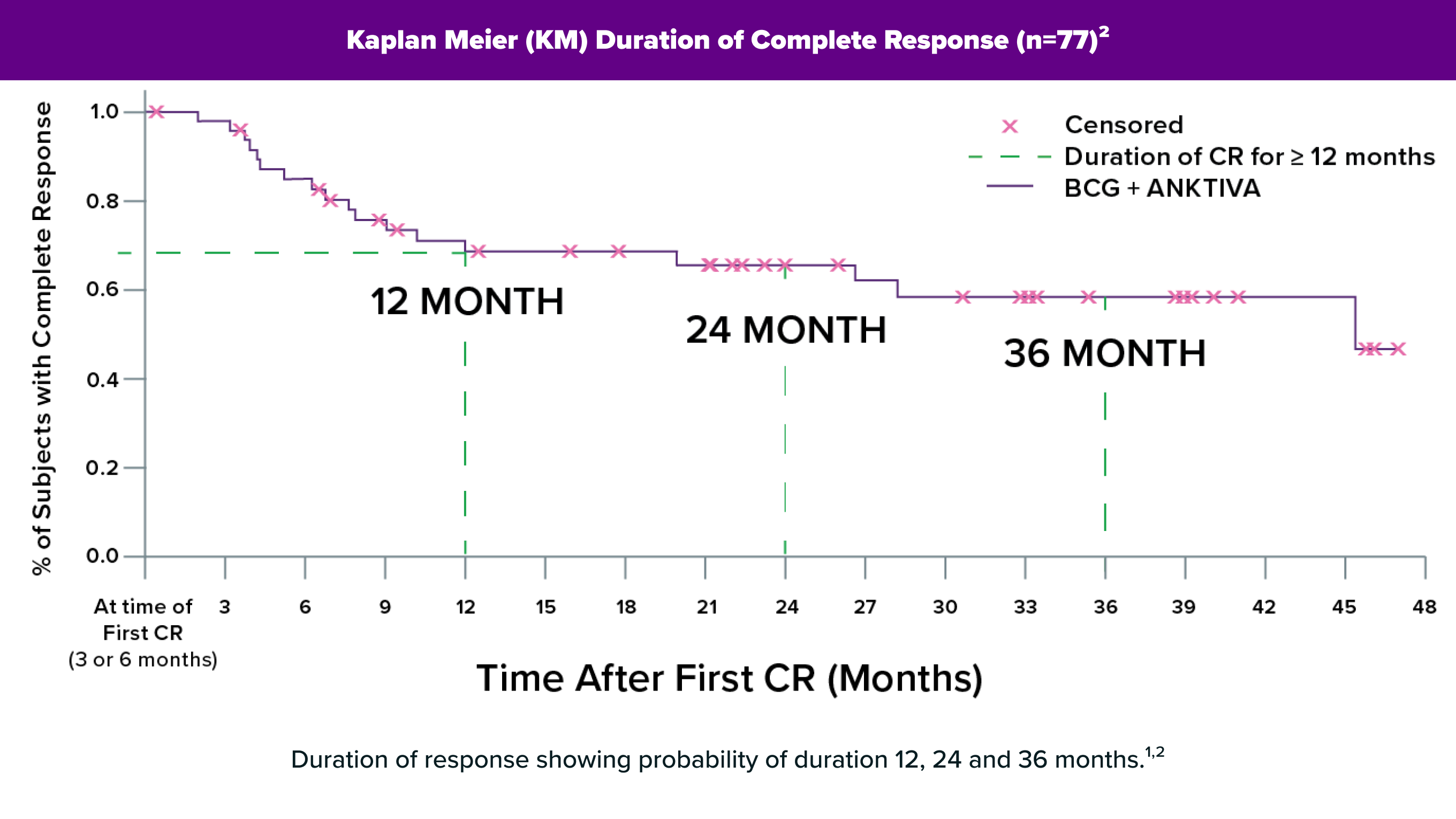
Treatment with BCG alone wasn’t effective. Why could using BCG with ANKTIVA be different?
ANKTIVA works differently and in concert with BCG. ANKTIVA’s immune-cell activator (interleukin-15) restores a response to BCG and increases the body’s disease-fighting NK cells, killer T cells, and memory T cells.1,2,3 ANKTIVA has been shown in studies to work with BCG to effectively kill cancer cells, when BCG alone wasn’t effective.4 And it has been shown to lead to longer duration responses.
How do I know if I am a good candidate for ANKTIVA?
It is important to discuss your options with your healthcare provider. They can help you evaluate whether a treatment involving ANKTIVA would be right for you.
1. Felices M, Chu S, Kodal B, Bendzick L, Ryan C, Lenvik AJ, Boylan KLM, Wong HC, Skubitz APN, Miller JS et al: IL-15 super-agonist (ALT-803) enhances natural killer (NK) cell function against ovarian cancer. Gynecol Oncol 2017, 145(3):453-461. 2. Van der Meer JMR, Maas RJA, Guldevall K, Klarenaar K, de Jonge P, Evert JSH, van der Waart AB, Cany J, Safrit JT, Lee JH et al: IL-15 superagonist N-803 improves IFNγ production and killing of leukemia and ovarian cancer cells by CD34(+) progenitor-derived NK cells. Cancer Immunol Immunother 2020, 70(11):3367. 3. Fantini M, David JM, Wong HC, Annunziata CM, Arlen PM, Tsang KY: An IL-15 Superagonist, ALT-803, Enhances Antibody-Dependent Cell-Mediated Cytotoxicity Elicited by the Monoclonal Antibody NEO-201 Against Human Carcinoma Cells. Cancer Biother Radiopharm 2019, 34(3):147-159. 4. Chamie K, Chang Sam S, Kramolowsky E, Gonzalgo Mark L, Agarwal Piyush K, Bassett Jeffrey C, Bjurlin M, Cher Michael L, Clark W, Cowan Barrett E et al: IL-15 Superagonist NAI in BCG-Unresponsive Non–Muscle-Invasive Bladder Cancer. NEJM Evidence 2022, 2(2200167):1-11.
ANKTIVA is a Novel Treatment for NMIBC CIS Patients Unresponsive to BCG
For more information, please call 1-877-ANKTIVA