ANKTIVA® is Co-Administered with BCG – for Familiar Storage and Dosing
Administration of ANKTIVA with BCG maintains the same favorable workflow and schedule as that of BCG alone in the urology practice environment
Preparation of BCG and ANKTIVA Admixture
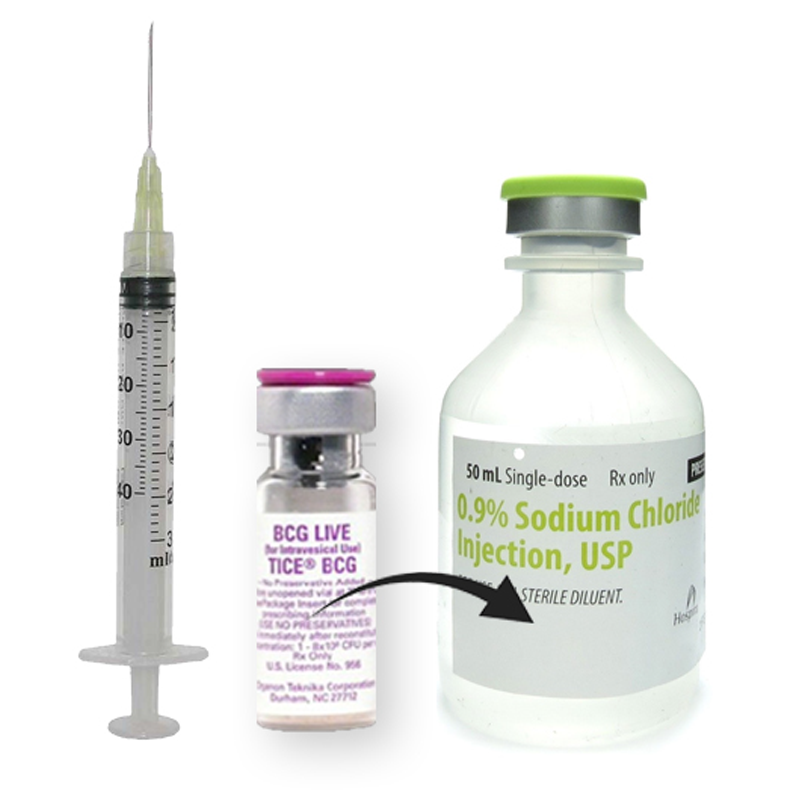
BCG Diluted in 50 mL Saline
Prepare BCG suspension following the instructions provided in the Prescribing Information for BCG with saline as follows:
Draw 1 mL of sterile, preservative-free saline (0.9% Sodium Chloride Injection USP) from a 50 mL vial of sterile saline at 4-25°C into a small syringe (e.g., 3 mL) and add to 1 vial of BCG to resuspend. Ensure that the needle is inserted through the center of the rubber stopper of the vial. Gently swirl the vial until a homogenous suspension is obtained. Avoid forceful agitation which may cause clumping of the mycobacteria.
Dilute the cloudy BCG suspension in the same 50 mL sterile, preservative-free saline vial to a final volume of 50 mL. Mix the suspension gently prior to step 2.
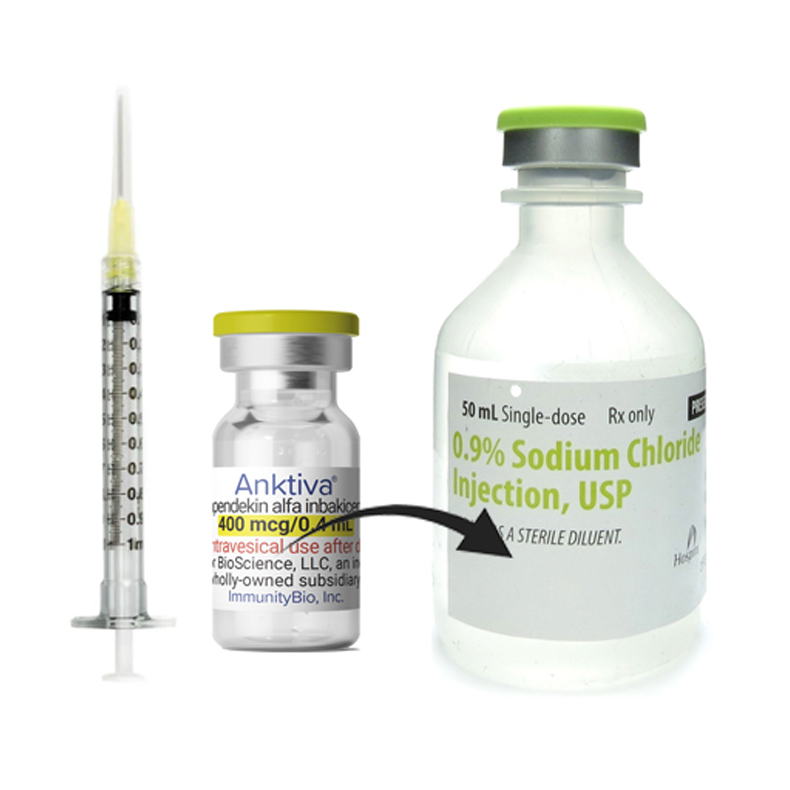
Anktiva Admixed in 50 mL Saline with BCG
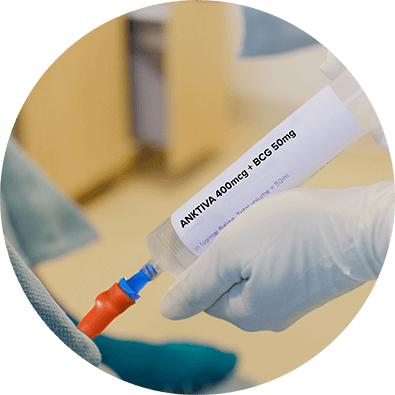
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. The solution is clear to slightly opalescent and colorless to slightly yellow. Discard the vial if visible particles are observed. Draw 0.4 mL of ANKTIVA into a small syringe and using aseptic technique add to the 50 mL saline volume containing the BCG suspension from step 1 that has been prepared following the instructions provided in the Prescribing Information for BCG.
Mix the suspension gently.
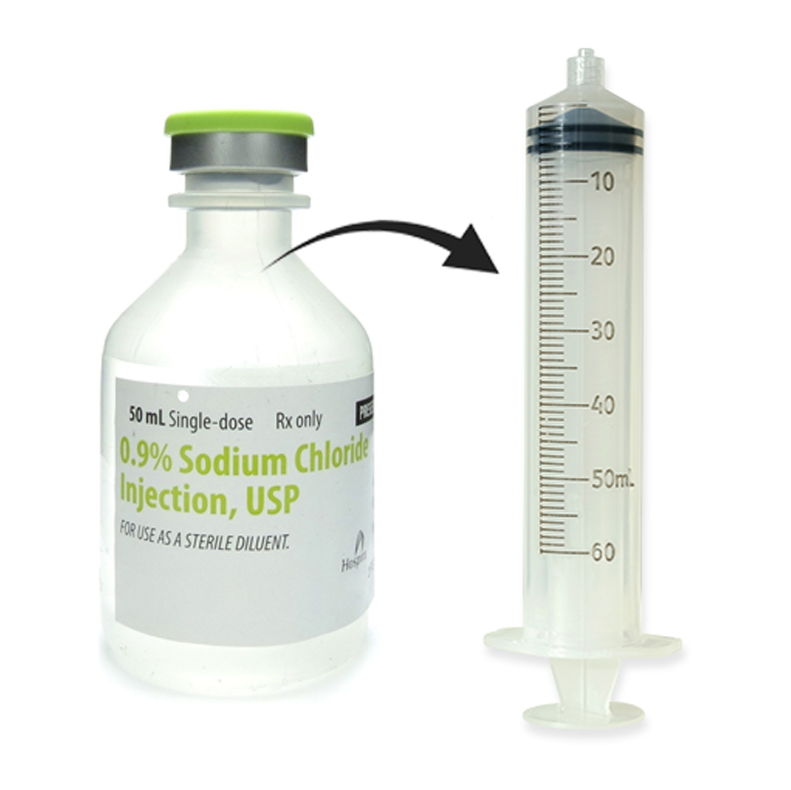
50 mL Admixed Volume Transferred to 60 mL Syringe
Using a 60-mL syringe connected to an appropriate size needle, withdraw the ANKTIVA BCG mixture to a final volume of 50 mL.
If the admixture of ANKTIVA in combination with BCG is not used immediately, store refrigerated at 2°C to 8°C (36°F to 46°F) and use within 2 hours. Unused solution of admixture should be discarded after 2 hours.
Intravesical Administration
Storage of ANKTIVA
ANKTIVA is stored at 2°C to 8°C (36°F to 46°F) and not frozen.
Do not shake.
Learn More About the Intravesical Instillation Schedule of ANKTIVA
FIRST INDUCTION
MAINTENANCE

FIRST & SECOND INDUCTION

MAINTENANCE


Preparation, Administration, and Storage
A summary of storage, preparation and administration, information for ANKTIVA
Patient Stories
Read about patients who participated in the clinical trials for ANKTIVA
Justin’s Journey
An avid outdoorsman, Justin faced a challenging diagnosis of non-muscle invasive bladder cancer. After struggling with the side effects of his Bacillus Calmette-Guérin (BCG) treatment, he found renewed hope though his doctors' introduction to an immunotherapy for those who experienced a recurrence of NMIBC.
Wayne’s Journey
Wayne has lived a rich life with his wife, two daughters, and three grandchildren. He describes himself as someone who “just likes people.” When he was first told, “you might have a tumor in your bladder,” he became very concerned, especially when he learned his first treatment with BCG wasn’t working. Wayne feels lucky that a friend told him about a clinical trial for patients like himself who were not cured by BCG alone.
ANKTIVA Resources
Information for ANKTIVA

ANKTIVA: Mechanism of Action
A description of how BCG and ANKTIVA work together in NMIBC CIS
ANKTIVA: How to Order
A step-by-step guide on how to order ANKTIVA, including product codes

Physicians' Journey
Listen to the physicians' journey with their patients using ANKTIVA.
ANKTIVA is a Novel Treatment for NMIBC CIS Patients Unresponsive to BCG
For more information, please call 1-877-ANKTIVA