About Non-Muscle Invasive Bladder Cancer

The standard of care for intermediate or high-risk non-muscle-invasive bladder cancer (NMIBC) patients is transurethral resection of the bladder tumor (TURBT) followed by Bacillus Calmette-Guérin (BCG) intravesical instillation. Yet up to 40% of these patients will have a recurrence despite these interventions. And half of patients who exhibit complete response will suffer a relapse.1
ANKTIVA® offers providers a powerful immunotherapy adjunct to BCG as a potential alternative to radical cystectomy and its long-term quality of life consequences for patients. ANKTIVA® plus BCG also can be administered by most urologists in their own clinics, without the need for special facilities or complex procedures.
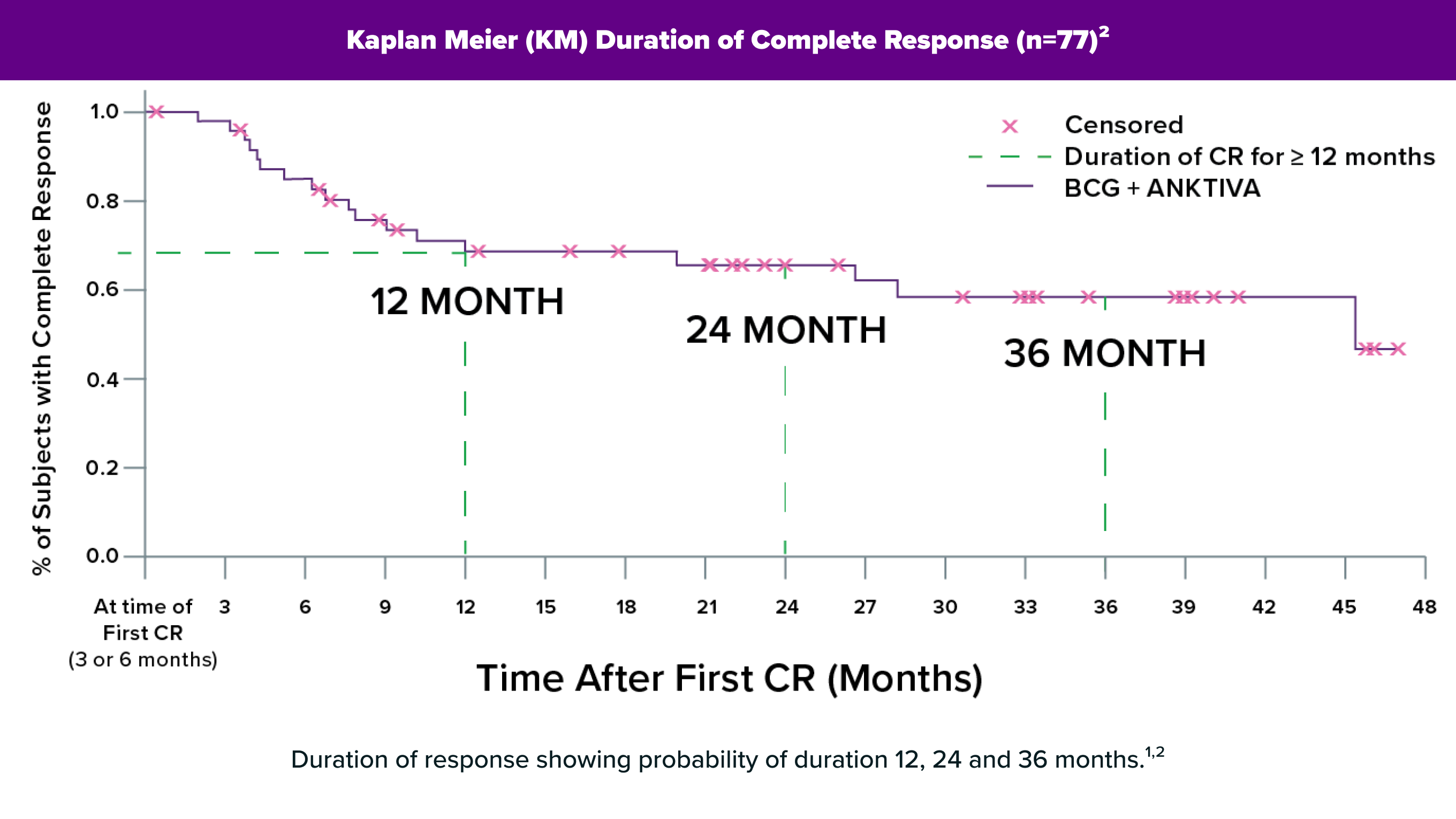
ANKTIVA® delivered in combination with BCG through intravesical instillation uses the power of the immune system to boost the efficacy of BCG, delivering a complete response with long-term durability in a majority of patients in a pivotal trial.
ANKTIVA plus BCG, when administered intravesically, is effective and safe.2
4 out of 5 people with bladder cancer have NMIBC.3 For an estimated 30-40 percent of patients, BCG therapy may not work long term.
1. Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 2011;49:466-477. DOI: 10.1016/j.eururo.2005.12.031. 2. ANKTIVA Package insert. lmmunityBio, Inc.; 2024. 3 Grabe-Heyne, K. et al. Intermediate and high-risk non-muscle-invasive bladder cancer: an overview of epidemiology, burden, and unmet needs. Frontiers in Oncology Jun 2 2023; 13: 1170124. doi: 10.3389/fonc.2023.1170124. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10272547/
ANKTIVA: How to Order
A step-by-step guide on how to order ANKTIVA, including product codes.
Patient Stories
Read about patients who participated in the clinical trials for ANKTIVA
Justin’s Journey
An avid outdoorsman, Justin faced a challenging diagnosis of non-muscle invasive bladder cancer. After struggling with the side effects of his Bacillus Calmette-Guérin (BCG) treatment, he found renewed hope though his doctors' introduction to an immunotherapy for those who experienced a recurrence of NMIBC.
Wayne’s Journey
Wayne has lived a rich life with his wife, two daughters, and three grandchildren. He describes himself as someone who “just likes people.” When he was first told, “you might have a tumor in your bladder,” he became very concerned, especially when he learned his first treatment with BCG wasn’t working. Wayne feels lucky that a friend told him about a clinical trial for patients like himself who were not cured by BCG alone.
ANKTIVA Resources
Information for ANKTIVA

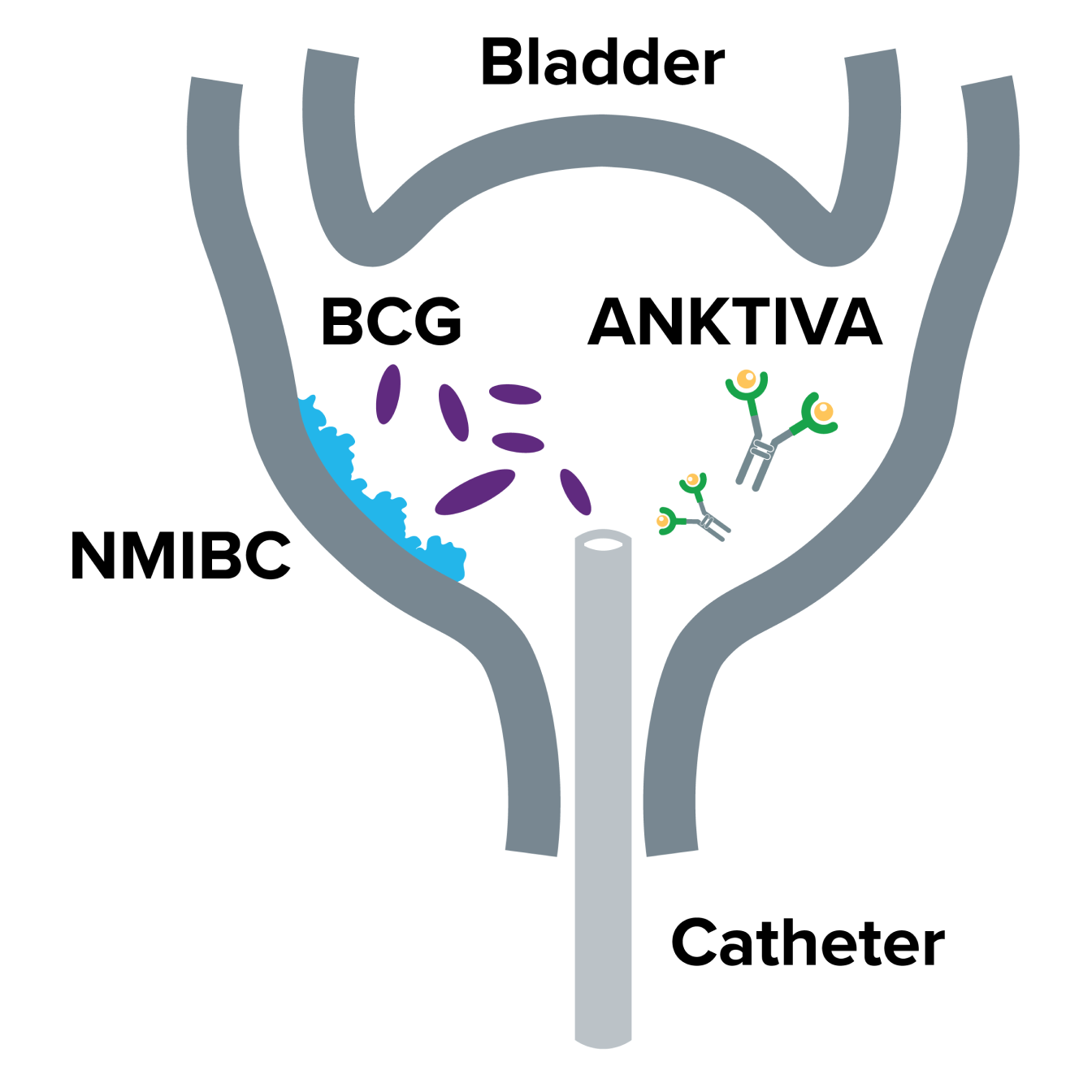
ANKTIVA: Mechanism of Action
A description of how BCG and ANKTIVA work together in NMIBC CIS

ANKTIVA: How to Order
A step-by-step guide on how to order ANKTIVA, including product codes

Physicians' Journey
Listen to the physicians' journey with their patients using ANKTIVA.
ANKTIVA is a Novel Treatment for NMIBC CIS Patients Unresponsive to BCG
For more information, please call 1-877-ANKTIVA