Resources
Healthcare Professionals Resources
ANKTIVA®: How to Order
A step-by-step guide on how to order ANKTIVA, including product codes
Download PDFANKTIVA®: Preparation, Administration, and Storage
A summary of storage, preparation and administration, information for ANKTIVA
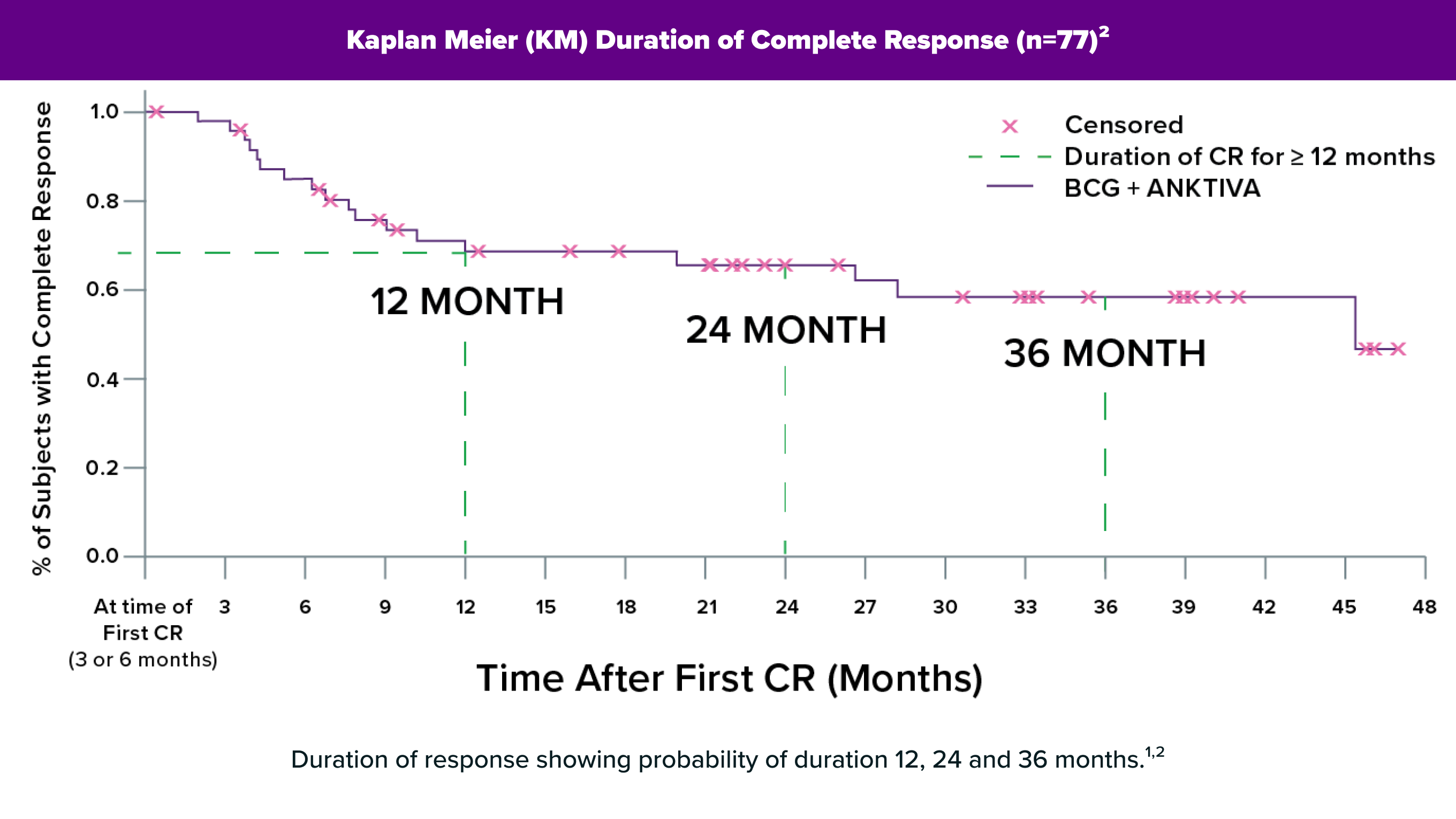
Download PDFANKTIVA®: Mechanism of Action
A description of how BCG and ANKTIVA work together in NMIBC CIS
Download PDFANKTIVA®: Access & Reimbursement Guide
Guidance on coverage, coding, and patient support for healthcare providers prescribing ANKTIVA®
Download PDFBCG + ANKTIVA Admixture Preparation Demonstration for Mixing Inside Saline Vial
A step-by-step video guide on preparation and administration of ANKTIVA for mixing inside saline vial
Watch VideoBCG + ANKTIVA Preparation Demonstration for Mixing Inside 50mL Syringe
A step-by-step video guide on preparation and administration of ANKTIVA inside of a 50 mL syringe
Watch VideoPatient Resources
Getting Started with ANKTIVA® for Patients
A patient-friendly overview of how ANKTIVA plus BCG work together, as well as what patients should expect before and after treatment
Download PDFFuture Oncology
A plain language review of the published findings on the Phase 1b BCG-naïve and Phase 2/3 BCG-unresponsive studies (QUILT 3.032)
Download PDFANKTIVA is a Novel Treatment for NMIBC CIS Patients Unresponsive to BCG
For more information, please call 1-877-ANKTIVA