ANKTIVA® Mechanism of Action (MOA): Harnessing Nature’s Immune System

ANKTIVA Synergizes with BCG Activating Innate (NK) and Adaptive (T Cell) Immune Memory – to Overcome T Cell Immune Evasion1,2
1. ANKTIVA Package insert. lmmunityBio, Inc.; 2024. 2. Suderman J, et al. Re: IL-15 Superagonist NAI in BCG Unresponsive Non-muscle-invasive Bladder Cancer. Eur Ural. 2023 Jun;83(6):581. doi: 10.1016/j.eururo. 2023.01.009.
Why ANKTIVA?
BCG treatment of NMIBC
BCG efficacy requires an effective immune response
Why try BCG again?
ANKTIVA restores the necessary immune response to BCG, including establishment of immune memory that leads to durable responses, by proliferating and activating NK and T cells.9,10,11
The interleukin-15 (IL-15) immune-cell activator ANKTIVA works synergistically with BCG to effectively kill cancer cells, even in BCG-unresponsive NMIBC patients for whom BCG alone was not effective.8
How effective is ANKTIVA plus BCG?
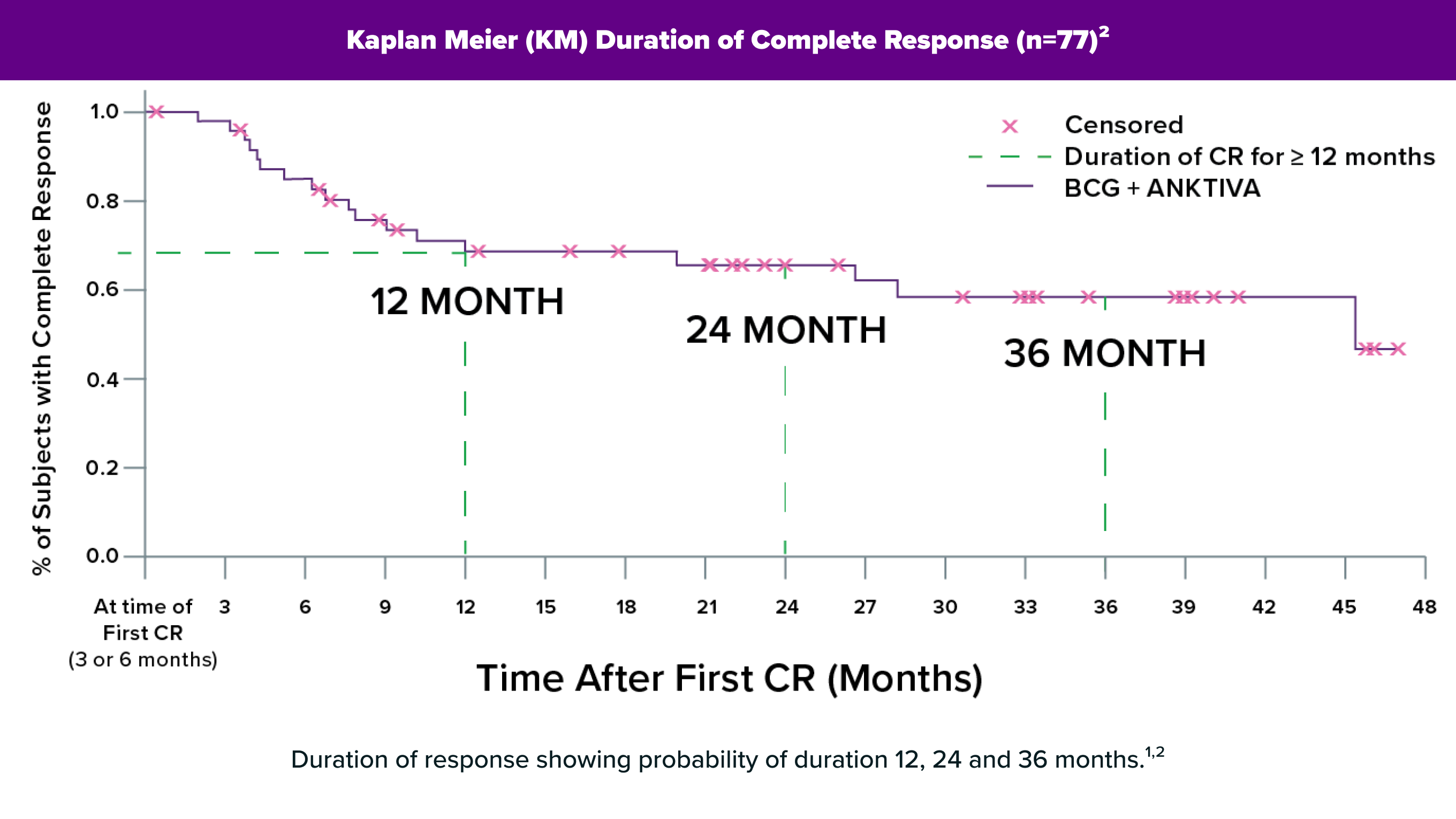
In the pivotal clinical trial, ANKTIVA plus BCG eliminated cancer in more than 62% of patients,12 with BCG-unresponsive bladder carcinoma in situ (CIS) with or without Ta/T1 papillary disease. These responses are typically durable, lasting more than a year in a majority of patients and more than 4 years in some patients, with many responses ongoing to date.
In the key clinical study QUILT 3.032, the response rate with ANKTIVA plus BCG was higher than that for other approved therapies for BCG-unresponsive bladder CIS, including both gene therapy or checkpoint inhibitor therapy.13
What is the practical advantage of ANKTIVA plus BCG?
The combination therapy is easily prepared before use and does not require extended thawing time, preventing waste, and is delivered following the familiar method and schedule as used for BCG alone.12
Because ANKTIVA is an intravesical therapy delivered directly to the bladder, the risk of systemic adverse reactions that occur with immunotherapies that have to be delivered by injection is very low. Thus, all the advantages of BCG therapy can be leveraged, with the increased efficacy provided by ANKTIVA, even in BCG-unresponsive patients.
1. American_Cancer_Society: Intravesical Therapy for Bladder Cancer. American Cancer Society website 2024, https://www.cancer.org/cancer/types/bladder-cancer/treating/intravesical-therapy.html (Accessed Nov 20, 2024). 2. Zlotta AR, Fleshner NE, Jewett MA: The management of BCG failure in non-muscle-invasive bladder cancer: an update. Can Urol Assoc J 2009, 3(6 Suppl 4):S199-205. 3. Sylvester RJ: Bacillus Calmette-Guérin treatment of non-muscle invasive bladder cancer. Int J Urol 2011, 18(2):113-120. 4. Brandau S, Riemensberger J, Jacobsen M, Kemp D, Zhao W, Zhao X, Jocham D, Ratliff TL, Bohle A: NK cells are essential for effective BCG immunotherapy. Int J Cancer 2001, 92(5):697-702. 5. Redelman-Sidi G, Glickman MS, Bochner BH: The mechanism of action of BCG therapy for bladder cancer–a current perspective. Nat Rev Urol 2014, 11(3):153-162. 6. Kates M, Matoso A, Choi W, Baras AS, Daniels MJ, Lombardo K, Brant A, Mikkilineni N, McConkey DJ, Kamat AM et al: Adaptive Immune Resistance to Intravesical BCG in Non-Muscle Invasive Bladder Cancer: Implications for Prospective BCG-Unresponsive Trials. Clin Cancer Res 2020, 26(4):882-891. 7. Lian J, Yue Y, Yu W, Zhang Y: Immunosenescence: a key player in cancer development. J Hematol Oncol 2020, 13(1):151. 8. Chamie K, Chang Sam S, Kramolowsky E, Gonzalgo Mark L, Agarwal Piyush K, Bassett Jeffrey C, Bjurlin M, Cher Michael L, Clark W, Cowan Barrett E et al: IL-15 Superagonist NAI in BCG-Unresponsive Non–Muscle-Invasive Bladder Cancer. NEJM Evidence 2023, Jan;2(1):EVIDoa2200167. 9. Felices M, Chu S, Kodal B, Bendzick L, Ryan C, Lenvik AJ, Boylan KLM, Wong HC, Skubitz APN, Miller JS et al: IL-15 super-agonist (ALT-803) enhances natural killer (NK) cell function against ovarian cancer. Gynecol Oncol 2017, 145(3):453-461. 10. Van der Meer JMR, Maas RJA, Guldevall K, Klarenaar K, de Jonge P, Evert JSH, van der Waart AB, Cany J, Safrit JT, Lee JH et al: IL-15 superagonist N-803 improves IFNγ production and killing of leukemia and ovarian cancer cells by CD34(+) progenitor-derived NK cells. Cancer Immunol Immunother 2021, 70(5):1305-1321. 11. Fantini M, David JM, Wong HC, Annunziata CM, Arlen PM, Tsang KY: An IL-15 Superagonist, ALT-803, Enhances Antibody-Dependent Cell-Mediated Cytotoxicity Elicited by the Monoclonal Antibody NEO-201 Against Human Carcinoma Cells. Cancer Biother Radiopharm 2019, 34(3):147-159. 12. FDA.gov: ANKTIVA Product Information. www.accessdata.fda.gov/drugsatfda_docs/label/2024/761336s000lbl.pdf. 13. Beinfeld M, Atlas SJ, Touchette D, McKenna A, Rind D, Pearson SD: The effectiveness and value of nadofaragene firadenovec, oportuzumab monatox, and pembrolizumab for BCG-unresponsive non-muscle-invasive bladder cancer. J Manag Care Spec Pharm 2021, 27(6):797-804.

Mechanism of Action (MOA)
A description of how BCG and ANKTIVA work together in NMIBC CIS
Patient Stories
Read about patients who participated in the clinical trials for ANKTIVA
Justin’s Journey
An avid outdoorsman, Justin faced a challenging diagnosis of non-muscle invasive bladder cancer. After struggling with the side effects of his Bacillus Calmette-Guérin (BCG) treatment, he found renewed hope though his doctors' introduction to an immunotherapy for those who experienced a recurrence of NMIBC.
Wayne’s Journey
Wayne has lived a rich life with his wife, two daughters, and three grandchildren. He describes himself as someone who “just likes people.” When he was first told, “you might have a tumor in your bladder,” he became very concerned, especially when he learned his first treatment with BCG wasn’t working. Wayne feels lucky that a friend told him about a clinical trial for patients like himself who were not cured by BCG alone.
ANKTIVA Resources
Information for ANKTIVA

ANKTIVA: Mechanism of Action
A description of how BCG and ANKTIVA work together in NMIBC CIS
ANKTIVA: How to Order
A step-by-step guide on how to order ANKTIVA, including product codes

Physicians' Journey
Listen to the physicians' journey with their patients using ANKTIVA.
ANKTIVA is a Novel Treatment for NMIBC CIS Patients Unresponsive to BCG
For more information, please call 1-877-ANKTIVA