Justin's Journey
An avid outdoorsman, Justin faced a challenging diagnosis of non-muscle invasive bladder cancer. After struggling with the side effects of his Bacillus Calmette-Guérin (BCG) treatment, he found renewed hope though his doctors’ introduction to an immunotherapy for those who experienced a recurrence of NMIBC.
Hearing the news
During a routine physical, a concerning finding in Justin’s urine, coupled with his family history of bladder cancer, prompted his physician to do further testing. It was then discovered that he had non-muscle invasive bladder cancer. Despite undergoing standard treatment with BCG, Justin faced a challenging journey with numerous symptoms, ultimately experiencing a recurrence.
Receiving Treatment
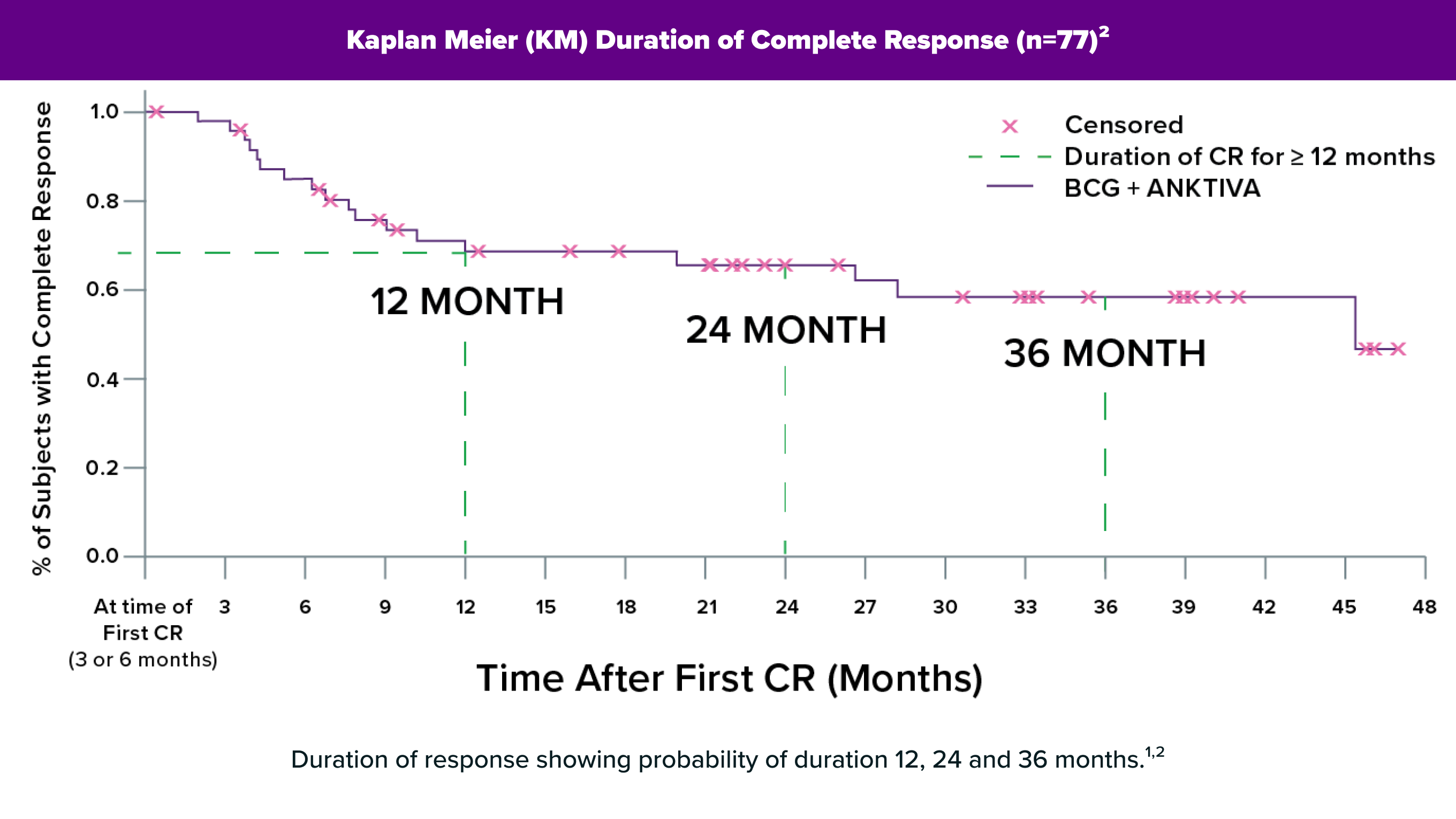
Justin felt relieved to be among the first patients in the country to receive ANKTIVA in combination with BCG, under the guidance of his physician, Dr. Chris Pieczonka. His response to treatment has been both durable and well-tolerated, without some of the complications he experienced during BCG monotherapy. He was able to quickly return to his regular activities, including working out and golfing, with minimal disruption. Since beginning treatment, Justin has had no disease recurrence or progression.
ANKTIVA is a Novel Treatment for NMIBC CIS Patients Unresponsive to BCG
For more information, please call 1-877-ANKTIVA