Think ANKTIVA®.
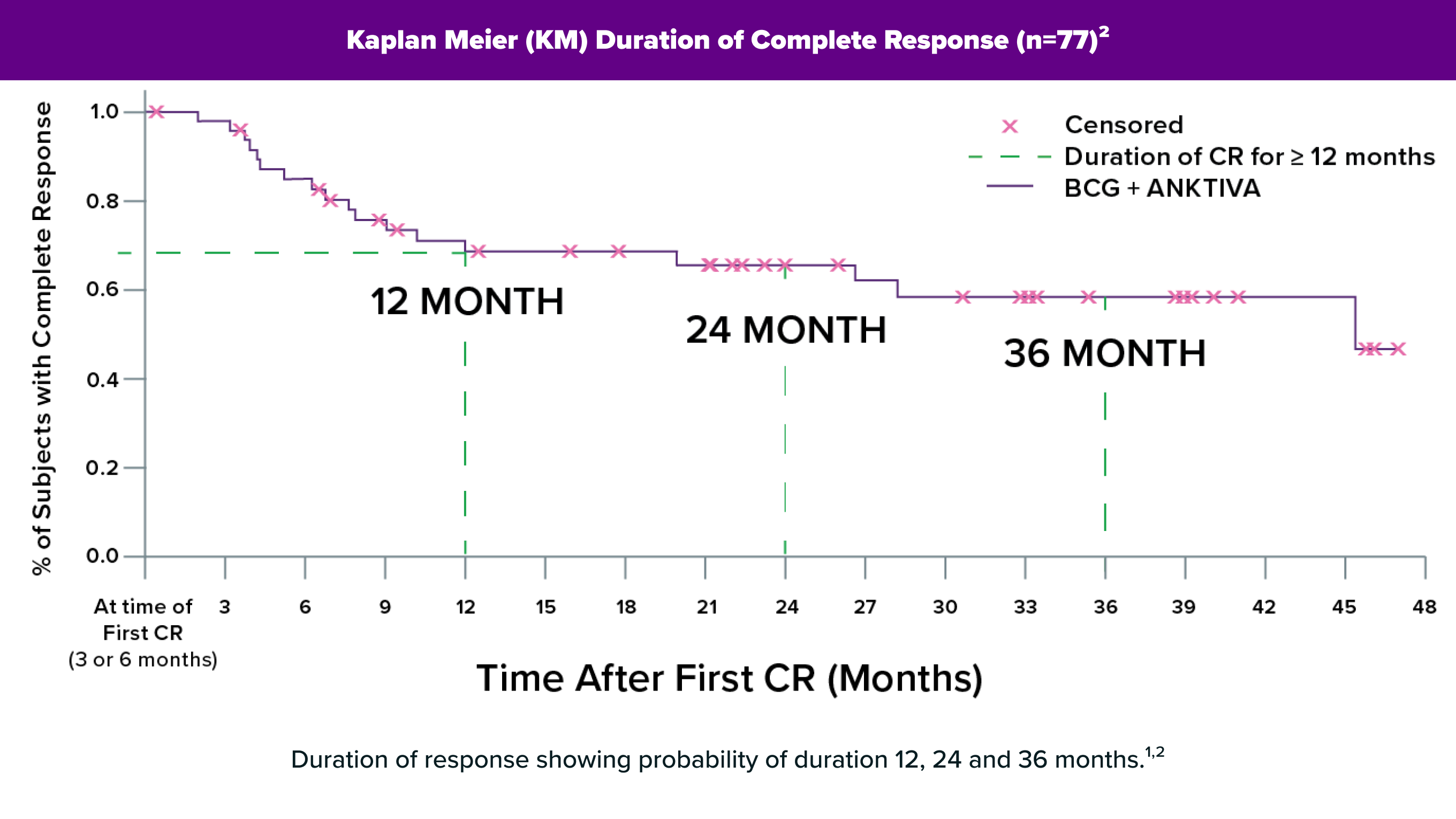
ANKTIVA is the first FDA-approved immunotherapy designed to activate the body’s natural immune system to target and attack BCG-unresponsive non-muscle invasive bladder CIS (NMIBC CIS), potentially leading to a long duration of complete response with some patients exceeding 53+ months1.

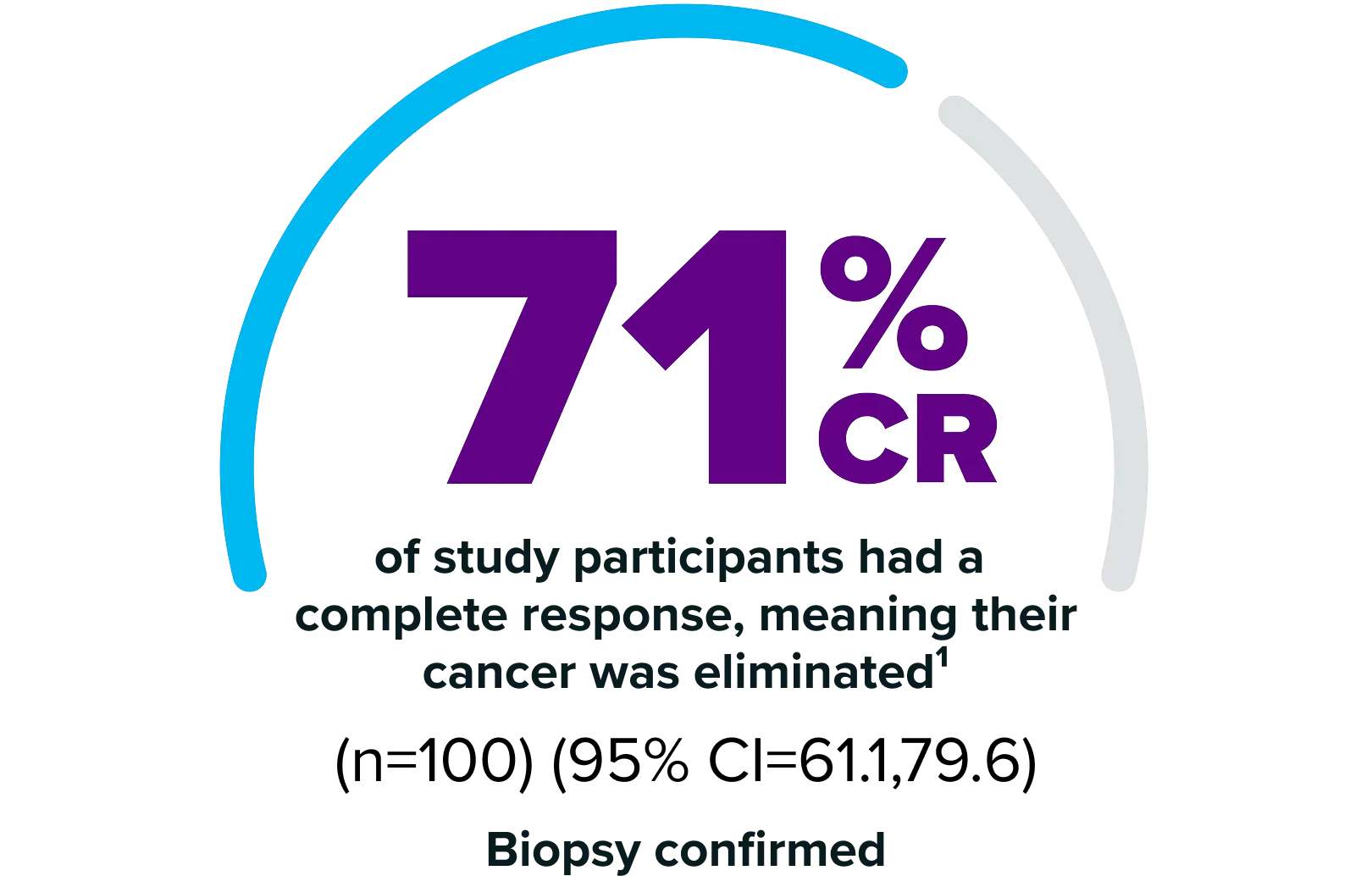
ANKTIVA is a Potent Immunostimulant that Unlocks Anti-tumor Activity and Unleashes Durable, Complete Responses.
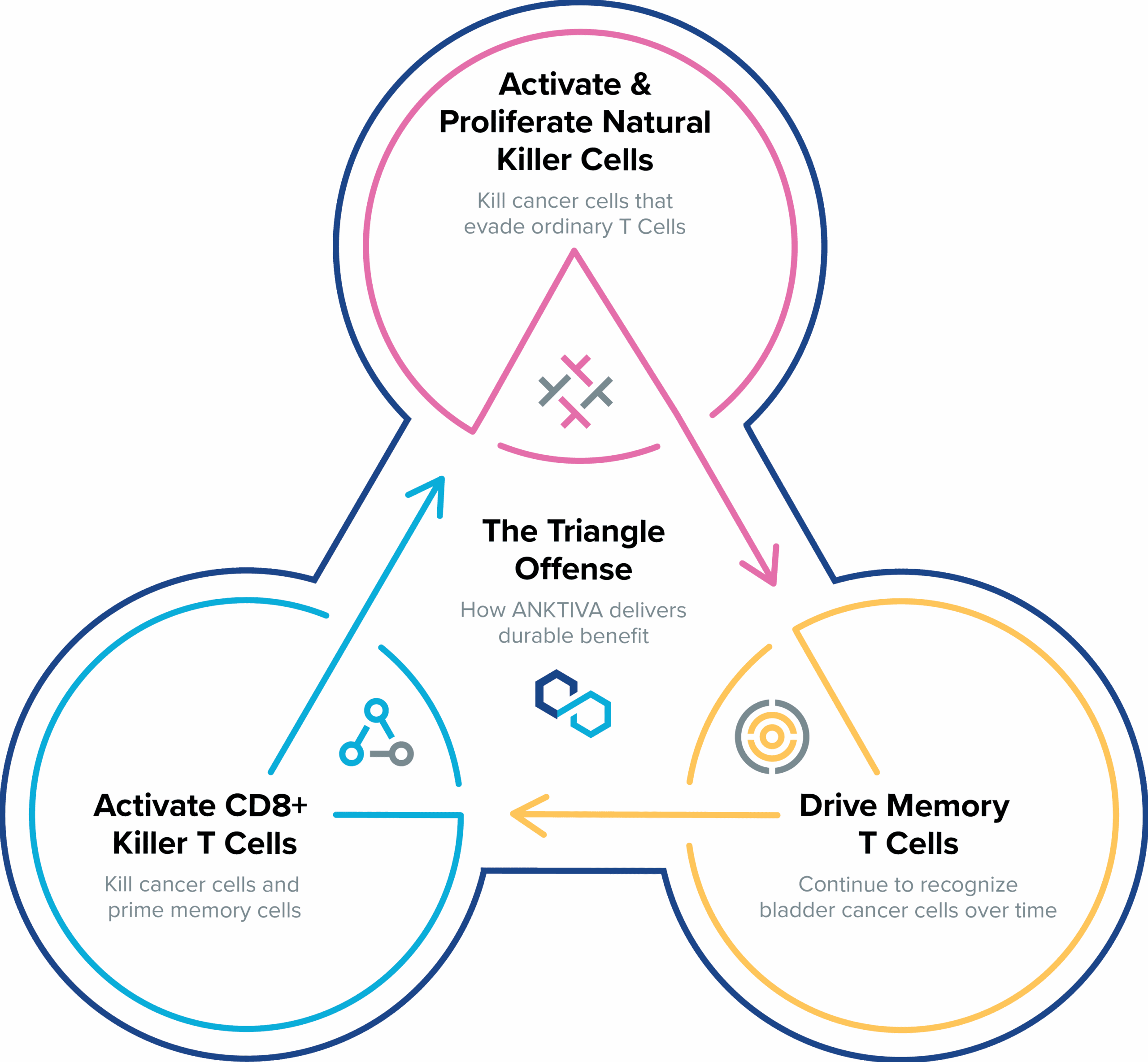
ANKTIVA uniquely stimulates the body’s natural immune system by proliferating and activating natural killer cells, as well as cytotoxic and memory T cells to drive long-term immune memory and durable responses.
Months
1. Chang, S. (2025, April 26-29). An Update on QUILT-3.032: Durable Complete Responses to NAI (ANKTIVA) Plus BCG Therapy in BCG-Unresponsive CIS With or Without Ta/T1 Papillary Disease and in Papillary Disease without CIS. [Conference Presentation]. AUA2025, Las Vegas, Nevada, United States.

Getting Started with ANKTIVA for Patients
A patient-friendly overview of how ANKTIVA plus BCG work together, as well as what patients should expect before and after treatment
Patient Stories
Read about patients who participated in the clinical trials for ANKTIVA
Justin’s Journey
An avid outdoorsman, Justin faced a challenging diagnosis of non-muscle invasive bladder cancer. After struggling with the side effects of his Bacillus Calmette-Guérin (BCG) treatment, he found renewed hope though his doctors' introduction to an immunotherapy for those who experienced a recurrence of NMIBC.
Wayne’s Journey
Wayne has lived a rich life with his wife, two daughters, and three grandchildren. He describes himself as someone who “just likes people.” When he was first told, “you might have a tumor in your bladder,” he became very concerned, especially when he learned his first treatment with BCG wasn’t working. Wayne feels lucky that a friend told him about a clinical trial for patients like himself who were not cured by BCG alone.
ANKTIVA Resources
Information for ANKTIVA

ANKTIVA: Mechanism of Action
A description of how BCG and ANKTIVA work together in NMIBC CIS
ANKTIVA: How to Order
A step-by-step guide on how to order ANKTIVA, including product codes

Physicians' Journey
Listen to the physicians' journey with their patients using ANKTIVA.
ANKTIVA is a Novel Treatment for NMIBC CIS Patients Unresponsive to BCG
For more information, please call 1-877-ANKTIVA