About Non-Muscle Invasive Bladder Cancer

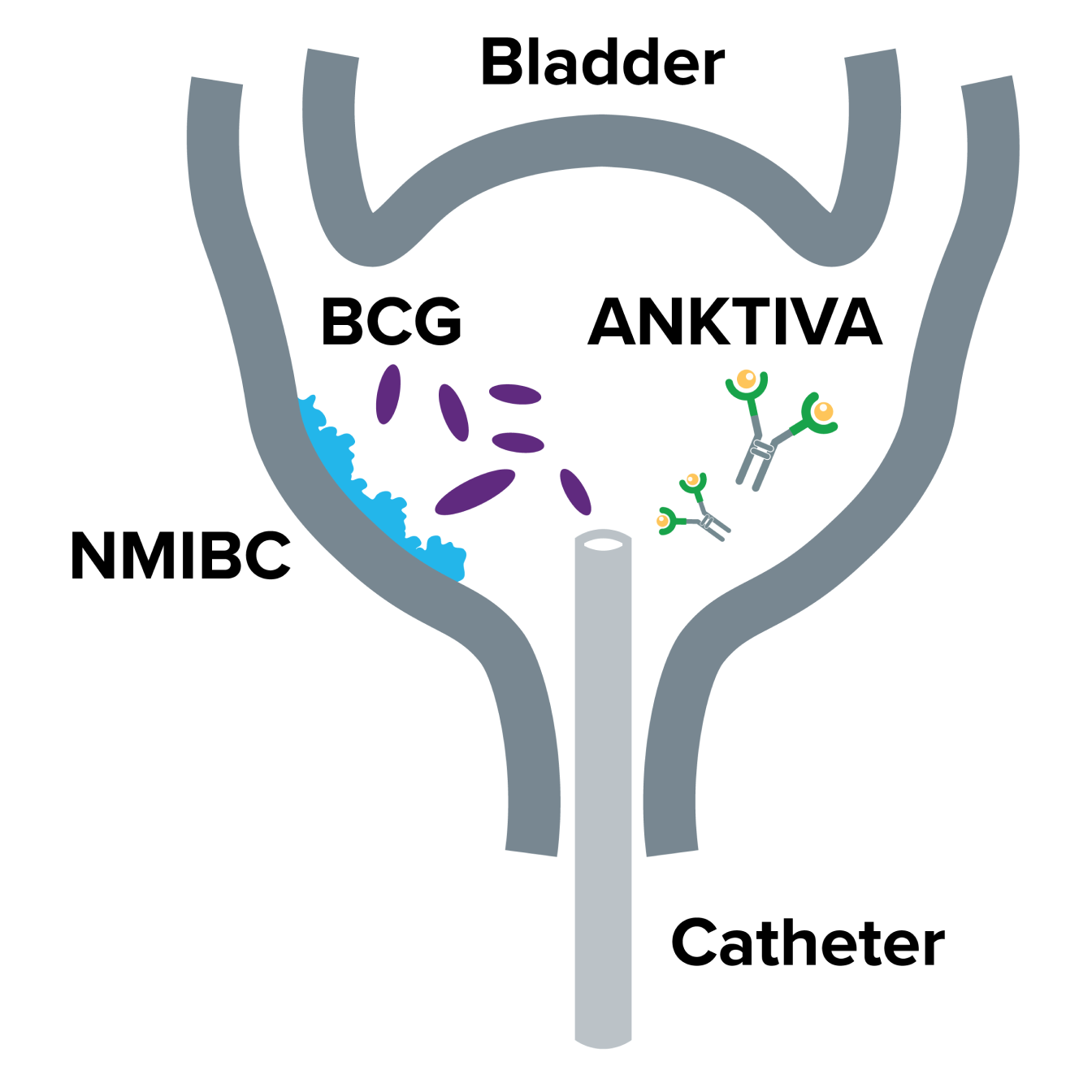
NMIBC is a type of cancer that grows on the inside lining of the bladder
The bladder is a hollow, balloon-like organ in which urine is stored. NMIBC, which is cancer that has grown only on the lining of the bladder and not into the muscle layer underneath, is the most common form of bladder cancer. NMIBC carcinoma in situ (CIS) is a critical subtype of NMIBC that is a high-grade, flat, and superficial form of bladder cancer.
Unlike other types of NMIBC that might form tumors or masses, CIS appears as a flat lesion on the bladder lining. It is characterized by abnormal cells that are confined to the bladder’s mucosal surface without invading the deeper layers of the bladder wall.
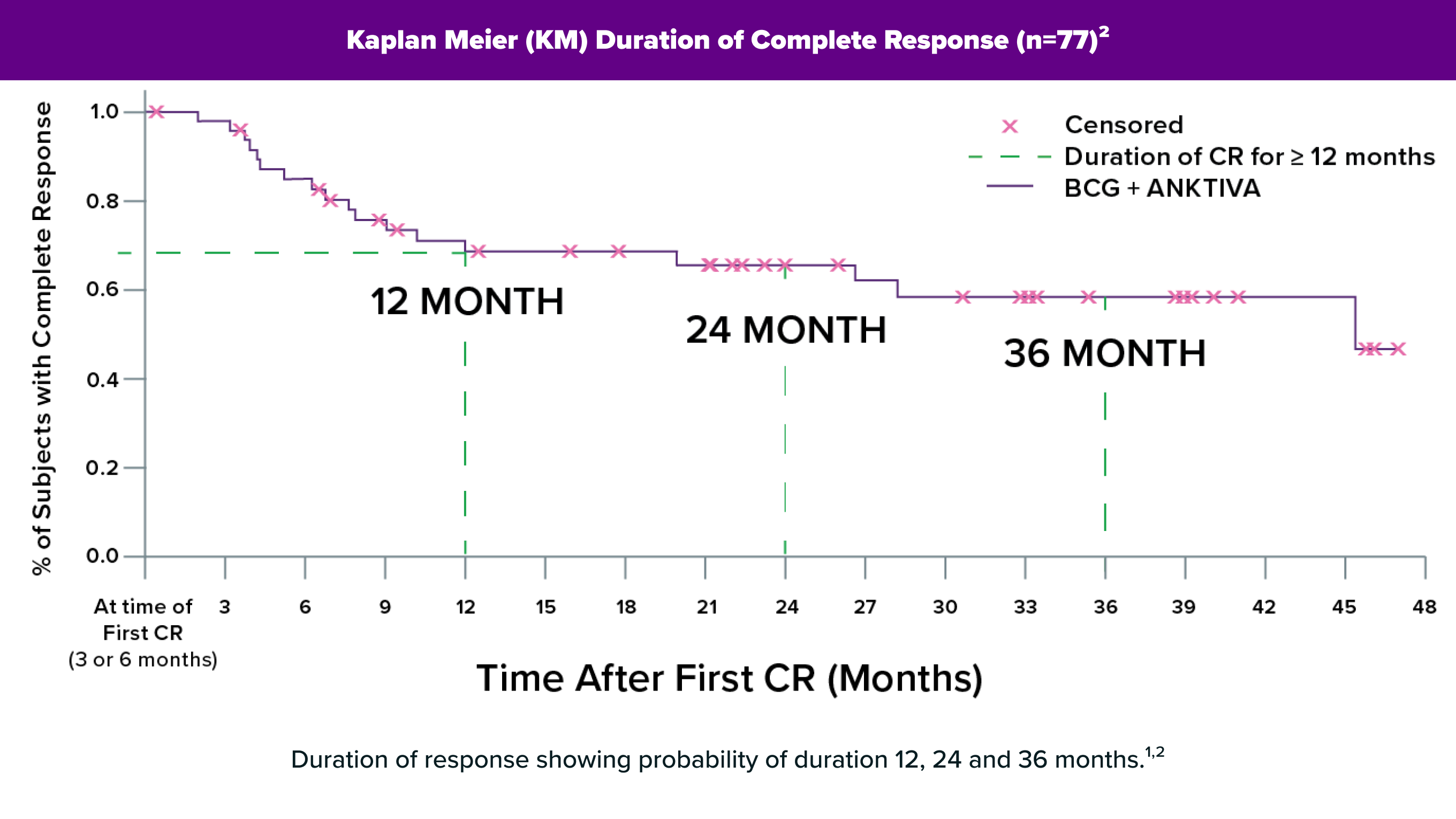
For NMIBC that is likely to keep growing (intermediate or high risk), BCG by itself is a common first therapy, and about 60% of patients with NMIBC will have a complete response.1,2 But in an estimated 30-40% of NMIBC patients, BCG will not work long-term, and the cancer will recur.2 For these BCG-unresponsive cases, there are only a few approved treatment options to try before surgical removal of the bladder (cystectomy) is recommended. But there is a reason for people with BCG-unresponsive NMIBC to feel hopeful – ANKTIVA® plus BCG – is an approved treatment option for BCG-unresponsive bladder CIS with or without Ta/T1 papillary disease that offers a chance for them to keep their bladder.
1. Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 2011;49:466-477. DOI: 10.1016/j.eururo.2005.12.031.

Getting Started with ANKTIVA for Patients
A patient-friendly overview of how ANKTIVA plus BCG work together, as well as what patients should expect before and after treatment
Patient Stories
Read about patients who participated in the clinical trials for ANKTIVA
Justin’s Journey
An avid outdoorsman, Justin faced a challenging diagnosis of non-muscle invasive bladder cancer. After struggling with the side effects of his Bacillus Calmette-Guérin (BCG) treatment, he found renewed hope though his doctors' introduction to an immunotherapy for those who experienced a recurrence of NMIBC.
Wayne’s Journey
Wayne has lived a rich life with his wife, two daughters, and three grandchildren. He describes himself as someone who “just likes people.” When he was first told, “you might have a tumor in your bladder,” he became very concerned, especially when he learned his first treatment with BCG wasn’t working. Wayne feels lucky that a friend told him about a clinical trial for patients like himself who were not cured by BCG alone.
ANKTIVA Resources
Learn more about the clinical story of ANKTIVA

Getting Started with ANKTIVA for Patients
A plain language review of the published findings on the Phase 1b BCG-naïve and Phase 2/3 BCG-unresponsive studies (QUILT 3.032).
ANKTIVA is a Novel Treatment for NMIBC CIS Patients Unresponsive to BCG
For more information, please call 1-877-ANKTIVA