How to Order
The ImmunityBio CARE™ program is designed to help answer questions about billing and coding for ANKTIVA® plus BCG, and determine if your patient’s insurance covers ANKTIVA, and assisting if it does not.
For more information contact 1-877-ANKTIVA (1-877-265-8482).
A Step-by-step Guide
STEP 1
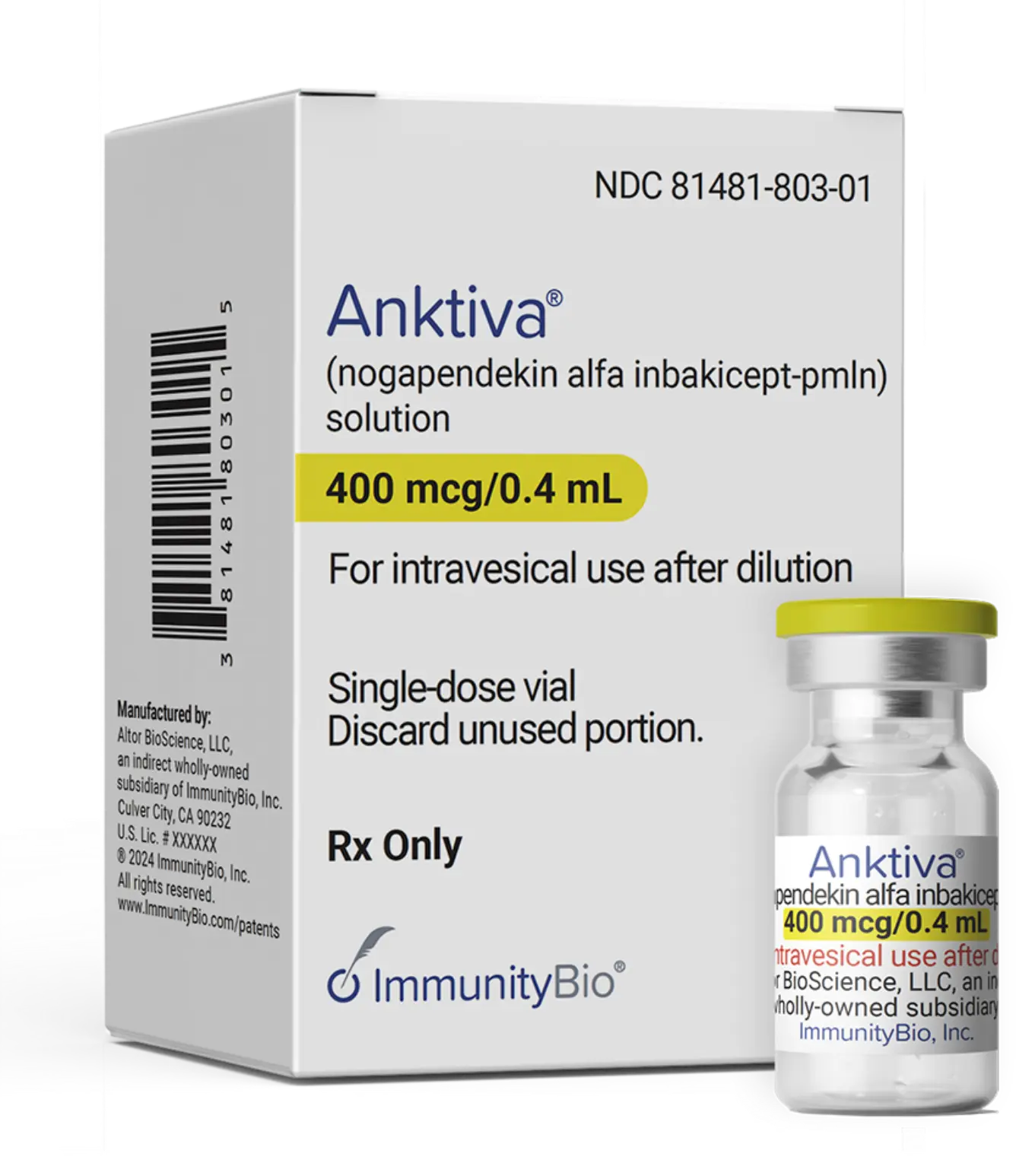
Ordering ANKTIVA®
| Product Information | |
|---|---|
| Name | ANKTIVA |
| Generic Name | nogapendekin alfa inbakicept-pmln |
| Package Presentation | Carton of one dose |
| Wholesale Acquisition Cost | $35,800 per dose |
| Dosage Form | Clear to slightly opalescent and colorless to slightly yellow solution in a single-dose vial |
| National Drug Code (NDC) | 1-vial package: NDC 81481-803-01 |
| Specialty Distributors |
Cencora (AmerisourceBergen) Oncology Supply: 800-633-7555 ASD: 800-746-6273 Besse Medical: 800-543-2111 McKesson McKesson Plasma & Biologics: 877-625-2566 McKesson Specialty Health: 800-482-6700 Cardinal Health 855-855-0708 CuraScript 877-599-7748 |
| Specialty Pharmacy |
Accredo 866-828-1129 www.accredo.com/prescribers/manage-referrals |
STEP 2: Coding & Reimbursement
Drug – First, Second Induction & Maintenance
| HCPCS J-Code | Healthcare Common Procedure Coding System (HCPCS) J-Code |
|---|---|
| J9028 | Anktiva® 400mcg, Code for Injection Services (Initial Code) *Please note: 400 mcg/0.4 mL single dose vial is equivalent to 400 billing units |
| 0636 | Anktiva® 400mcg, Drug Required Revenue Code for HOPD via OPPS |
| J9030 | BCG Live lntravesical Instillation, 1mg |
| A9589 | Blue Light Instillation, Hexaminolevulinate Hydrochloride, 100 mg |
| C9738 | Adjunctive Blue Light Cystoscopy with Fluorescent Imaging Agent |
Diagnosis & Findings
| ICD-10 | International Coding For Diseases (ICD) Description |
|---|---|
| C67.0 | Malignant Neoplasm: Trigone of Bladder |
| C67.1 | Malignant Neoplasm: Dome of Bladder |
| C67.2 | Malignant Neoplasm: Lateral Wall of Bladder |
| C67.3 | Malignant Neoplasm: Anterior Wall of Bladder |
| C67.4 | Malignant Neoplasm: Posterior Wall of Bladder |
| C67.5 | Malignant Neoplasm: Bladder Neck |
| C67.6 | Malignant Neoplasm: Ureteric Orifice |
| C67.7 | Malignant Neoplasm: Urachus |
| C67.8 | Malignant Neoplasm: Overlapping Lesion of Bladder |
| C67.9 | Malignant Neoplasm: Bladder – Unspecified |
| D09.0 | Carcinoma In Situ of Bladder |
| R82.7 | Abnormal Findings on Microbiological Examination of Urine |
| R82.8 | Abnormal Findings on Cytological & Histological Examination of Urine |
| R82.9 | Other and Unspecified Abnormal Findings in Urine |
| Z85.51 | Personal History of Malignant Neoplasm of Bladder |
| Z85.52 | Family History of Malignant Neoplasm of Bladder |
Procedure: 1st Induction, 2nd Induction, Maintenance & Follow-up (Up to 5 Years)
| CPT Code | Current Procedural Terminology (CPT) Description |
|---|---|
| 51720 | Bladder Instillation of Anticarcinogenic Agent, Including Retention Time |
| 52000 | Endoscopy-Cystoscopy, Urethroscopy, Cystourethroscopy Procedures on the Bladder |
| 52204 | Urethra & Bladder Transurethral Surgical Procedures |
| 52234 | Cystourethroscopy, with Fulguration, Including Cryosurgery or Laser Surgery, and/or Resection of Small Bladder Tumor(s) (0.5 up to 2.0 cm) |
| 52235 | Cystourethroscopy, with Fulguration, Including Cryosurgery of LaserSurgery, and/or Resection of Medium Bladder Tumor(s) (2.0 to 5.0cm) |
| 52240 | Cystourethroscopy, with Fulguration, Including Cryosurgery of Laser Surgery, and/or Resection of Large Bladder Tumor(s) (>5.0cm) |
| 88104 | Cytopath Nongynological Smear |
| 88108 | Cytopath Concentrate Technical |
| 88112 | Cytopath Cell Enhance Technical |
Office Visit
| E/M Code | Evaluation and Management Description |
|---|---|
| 99203 | New Patient Office or Other Outpatient Visit, 30-44 Minutes |
| 99204 | New Patient Office or Other Outpatient Visit, 45-59 Minutes |
| 99213 | Established Patient Office or Other Outpatient Visit, 20-29 Minutes |
| 99214 | Established Patient Office or Other Outpatient Visit, 20-29 Minutes |
Billing Information Sheet
Anktiva® National Drug Code (NDC): 81481-803-01
Clinic: Freestanding Clinic Reimbursement is Based on Local MAC Payment Methodology
Hospital: Hospital Outpatient Prospective Payment System, Pass-Through Payment In-Process
Disclaimer
The billing and coding information in this guide is for general informational purposes only. This should not be relied upon for purposes of determining payer coverage and coding. This information represents no promise, commitment, statement or guarantee by ImmunityBio concerning proper billing or coding practices or levels of reimbursement, payment, or charges. The materials referenced and provided are based upon coding experience and research of current general coding practices. The existence of codes does not guarantee coverage or payment for any procedure by any payer. The final decision for coding of any procedure must be made by the provider of care after considering the medical necessity of the services and supplies provided as well as the regulations and local, state, or federal laws that may apply.
The information contained in this guide is provided to help you understand the reimbursement information and is not intended to suggest any way you can increase or maximize reimbursement from any payer. Reimbursement information is gathered from third-party sources and is subject to change. We recommend that you consult with payers for specific coverage and billing requirements.
All Current Procedural Terminology (CPT®) codes, Healthcare Common Procedural Coding System (HCPCS) codes, Ambulatory Payment Classifications (APCs) and National Drug Codes (NDC) are provided for your information only and ImmunityBio does not represent that these codes are or will be appropriate or that reimbursement will be made if using them or any other codes. CPT® codes and descriptions only are copyrighted by the American Medical Association (AMA). CPT®, APC and other codes do not include fee schedules, relative values, or related listings. The Centers for Medicare & Medicaid Services (CMS) updates coverage, coding, and payment information frequently, and it is the responsibility of each health service provider to confirm the appropriate billing required by the local Medicare contractor.
Providers should refer to authoritative coding sources, such as the CPT® codes and HCPCS codes. Applicable FARS/DFARS Restrictions Apply to Government Use. Fee schedules, relative value units, conversion factors and/or related components are not assigned by the AMA, are not part of CPT®, and the AMA is not recommending their use. The AMA does not directly or indirectly practice medicine or dispense medical services. The AMA assumes no liability for data contained or not contained herein. The information provided in this guide is for informational purposes only. Information included does not guarantee coverage or payment. Payment will vary by geographic locality. It is always the provider’s responsibility to determine coding and claims information for the services that were provided.
STEP 3: lntravesical Instillation Schedule of ANKTIVA®
FIRST INDUCTION
ANKTIVA 400 mcg administered intravesically with BCG once a week for 6 weeks. A second induction course is not required if a complete response is achieved at month 3.
MAINTENANCE

For Maintenance: After BCG and ANKTIVA induction therapy, ANKTIVA is recommended at a dose of 400 mcg administered intravesically with BCG once a week for 3 weeks at months 4, 7, 10, 13 and 19 (for a total of 15 doses). For patients with an ongoing complete response at month 25 and later, maintenance instillations may be administered once a week for 3 weeks at months 25, 31, and 37 for a maximum of 9 additional instillations.
FIRST & SECOND INDUCTION

ANKTIVA 400 mcg administered intravesically with BCG once a week for 6 weeks. A second induction course is not required if a complete response is achieved at month 3.
MAINTENANCE

For Maintenance: After BCG and ANKTIVA induction therapy, ANKTIVA is recommended at a dose of 400 mcg administered intravesically with BCG once a week for 3 weeks at months 7, 10, 13 and 19 (for a total of 15 doses). For patients with an ongoing complete response at month 25 and later, maintenance instillations may be administered once a week for 3 weeks at months 25, 31, and 37 for a maximum of 9 additional instillations.
1. Chamie K, et al. IL-15 Superagonist NAI in BCG-Unresponsive Non-Muscle-Invasive Bladder Cancer. NEJM Evid. 2023 Jan;2(1): EVIDoa2200167. doi: 10.1056/EVIDoa2200167.
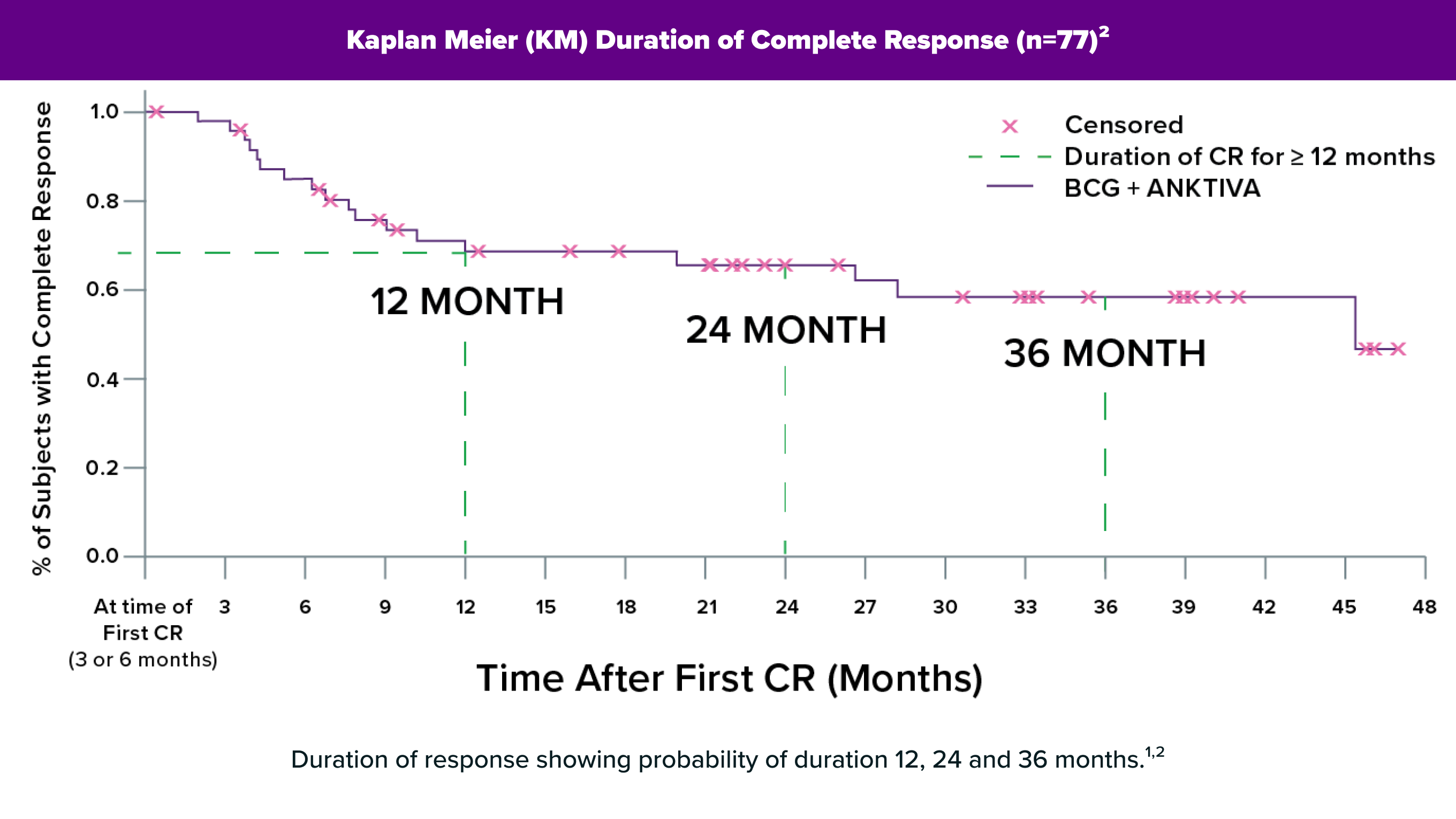
ANKTIVA is a Novel Treatment for NMIBC CIS Patients Unresponsive to BCG
For more information, please call 1-877-ANKTIVA