Irv's Journey
Irv is a dentist who loves to travel, particularly with his wife to see his grown children and seven grandkids – including identical twins. “Family is the number one thing in life. I can’t stand being without the grandkids,” Irv says, “I monopolize them. A 900 billion dollar lottery win couldn’t be better.”
Hearing the news
He was first diagnosed with bladder cancer in 2016 when on Thanksgiving day he had very concerning symptoms, including blood in his urine, and several tumors on the interior surface of his bladder were removed by his local urologist, who treated him with BCG, but he failed to respond and the tumors came back. He was afraid he was going to lose his bladder.
Participating in ImmunityBio’s QUILT 3.032 study
It was one one Irv’s dental patients who encouraged him to contact Dr. Sam Chang at Vanderbilt University, who described the Anktiva plus BCG trial. Irv feels fortunate now that he sold his dental practice just before the pandemic, because it allowed him to easily participate in the trial. And he did – driving 400 miles roundtrip over the early months of 2020 to receive treatment and assessment of his bladder cancer. It was worth it. He did not have any tumor recurrence over the two years of the study. At first, he didn’t understand why BCG was being used again, but learned Anktiva was designed to enhance BCG effects, boosting his own cancer-fighting natural killer and T cells.
He says his positivity has returned. “Cheers and happy times again!” and with it, at 70, running about six miles a day. He remembers the moment even his urine was free of cancer cells, “woo-hoo!”
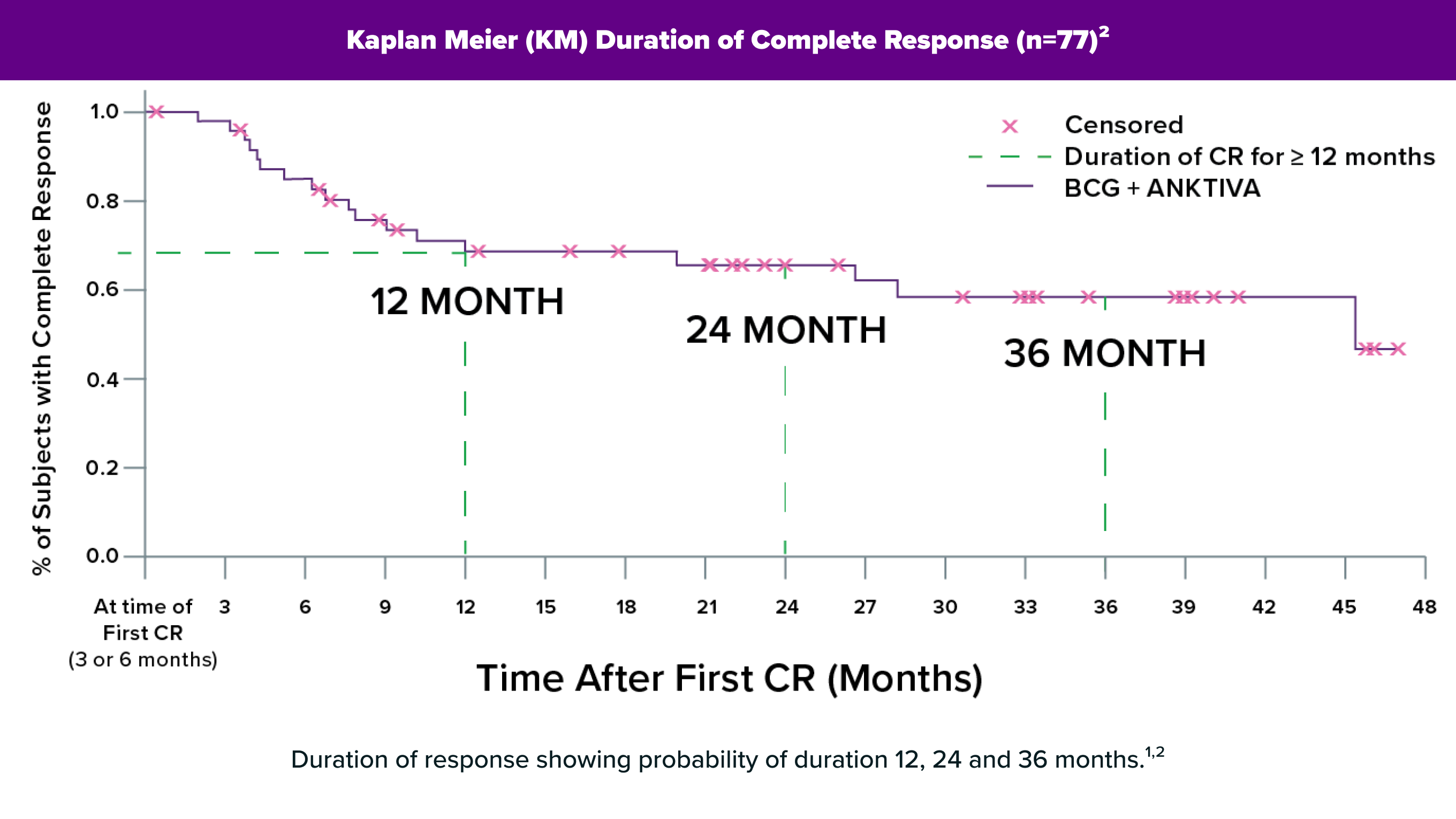
ANKTIVA is a Novel Treatment for NMIBC CIS Patients Unresponsive to BCG
For more information, please call 1-877-ANKTIVA