With cancer, time matters.
Designated an FDA Breakthrough Therapy for Non-Muscle Invasive Bladder Cancer, the novel immunotherapy ANKTIVA® activates key killer cells of the body’s natural immune system to attack the bladder cancer, leading to a long-duration of complete response that for some patients exceeds 47 months.1

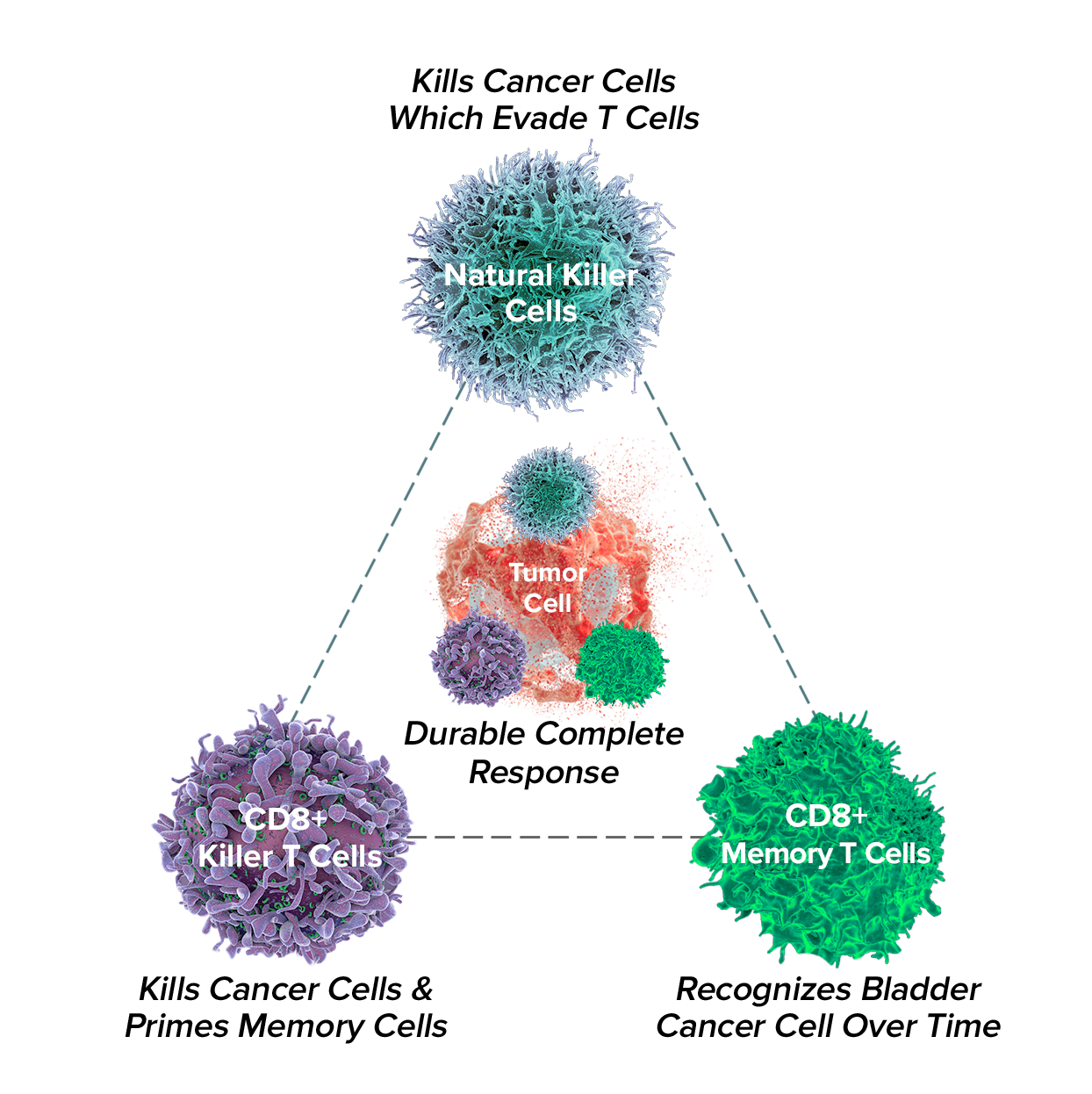
ANKTIVA is a potent immunostimulant that unlocks anti-tumor activity and promotes a durable, complete response.
ANKTIVA works by activating the body’s natural immune system to fight disease and provide long-term immune memory against the cancer.
Duration of Complete Response
Maintenance Therapy
1. ANKTIVA Package insert. lmmunityBio, Inc.; 2024.
The Power of ANKTIVA
ANKTIVA is an IL-15 receptor alpha that generates IL-15-activated NK, Killer T, and Memory T Cells while avoiding T-reg stimulation
Safety
& Tolerability
Intravesical
Administration
Efficacy
Profile
| 62% | 71% |
|
N=771
(FDA Efficacy Population)
|
N=822
(NEJM Evidence)
|
Prolonged Duration
of Response
& Ongoing
1. ANKTIVA Package insert. lmmunityBio, Inc. ; 2024. 2. Chamie K, et al. IL-15 Superagonist NAI in BCG-Unresponsive Non-Muscle-Invasive Bladder Cancer. NEJM Evid. 2023 Jan;2(1): EVIDoa2200167. doi: 10.1056/EVIDoa2200167. 3. Suderman J, et al. Re: IL-15 Superagonist NAI in BCG Unresponsive Non-muscle-invasive Bladder Cancer. Eur Ural. 2023 Jun;83(6):581. doi: 10.1016/j.eururo. 2023.01.009. 4. BCG Package insert. Merck & Co., Inc. ; 2022. 5. Chamie K, et al. Quality of Life in the Phase 2/3 Trial of N-803 Plus Bacillus Calmette-Guerin in Bacillus Calmette-Guerin Unresponsive Nonmuscle-lnvasive Bladder Cancer. Ural Pract. 2024 Mar;11(2):367-375. doi: 10.1097/UPJ.0000000000000517. 6. Han KP, et al.. IL-15:IL-15 receptor alpha superagonist complex: high-level co-expression in recombinant mammalian cells, purification and characterization. Cytokine. 2011 Dec;56(3):804-10. doi: 10.1016/j.cyto.2011.09.028. 7. One death reported due to a cardiac arrest that was unrelated to N-803 + BCG. 8. Kamat AM, Sylvester RJ, Bbhle A, Palau J, Lamm DL, Brausi M, Soloway M, Persad R, Buckley R, Colombel M, Witjes JA. Definitions, End Points, and Clinical Trial Designs for Non-Muscle-Invasive Bladder Cancer: Recommendations From the International Bladder Cancer Group. J Clin Oneel. 2016 Jun 1;34(16):1935-44. doi: 10.1200/ JCO.2015.64.4070.
Patient Stories
Read about patients who participated in the clinical trials for ANKTIVA.
Irv's Journey
He was first diagnosed with bladder cancer in 2016 when on Thanksgiving day he had very concerning symptoms, including blood in his urine, and several tumors on the interior surface of his bladder were removed by his local urologist, who treated him with BCG, but he failed to respond and the tumors came back. He was afraid he was going to lose his bladder.
Wayne’s Journey
Wayne has lived a rich life that includes his wife, two daughters and three grandkids. He describes himself as someone who “just likes people.”

ANKTIVA® is a new choice to help conquer
non-muscle invasive bladder cancer.
For more information, please call 1-877-ANKTIVA
